Results for “rapid test” 292 found
Combination Rapid Tests
Once again, the US is behind on at-home rapid antigen tests–this time on combination tests that let you test for COVID, Influenza, and RSV all at once. These tests are widely available in Europe but have not been approved by the FDA. Rapid flu tests especially are potentially very useful in assigning appropriate treatment and reducing the overuse of antibiotics.
ProPublica on the FDA and Rapid Tests
Lydia DePillis has written the best piece on the FDA that I have ever read in a mainstream news publication. It gets everything right and yes it frankly verifies everything that I have been saying about the FDA and rapid tests for the last year and a half. I wish it had been written earlier but I suppose that illustrates how difficult it is to radically change people’s mindset from the FDA as protector to the FDA as threat. The sub head is:
Irene Bosch developed a quick, inexpensive COVID-19 test in early 2020. The Harvard-trained scientist already had a factory set up. But she was stymied by an FDA process experts say made no sense.
The piece recounts how cheap, rapid tests could have been approved in March of 2020! Here’s the opening bit:
When COVID-19 started sweeping across America in the spring of 2020, Irene Bosch knew she was in a unique position to help.
The Harvard-trained scientist had just developed quick, inexpensive tests for several tropical diseases, and her method could be adapted for the novel coronavirus. So Bosch and the company she had co-founded two years earlier seemed well-suited to address an enormous testing shortage.
E25Bio — named after the massive red brick building at MIT that houses the lab where Bosch worked — already had support from the National Institutes of Health, along with a consortium of investors led by MIT.
Within a few weeks, Bosch and her colleagues had a test that would detect coronavirus in 15 minutes and produce a red line on a little chemical strip. The factory where they were planning to make tests for dengue fever could quickly retool to produce at least 100,000 COVID-19 tests per week, she said, priced at less than $10 apiece, or cheaper at a higher scale.
“We are excited about what E25Bio is capable of shipping in a short amount of time: a test that is significantly cheaper, more affordable, and available at-home,” said firm founder Vinod Khosla. (Disclosure: Khosla’s daughter Anu Khosla is on ProPublica’s board.)
On March 21 — when the U.S. had recorded only a few hundred COVID-19 deaths — Bosch submitted the test for emergency authorization, a process the Food and Drug Administration uses to expedite tests and treatments.
You know how the story ends but really READ the WHOLE THING.
The Slow Rollout of Rapid Tests
I thought the Biden administration would at least make original pandemic errors. But no, its been making all the same errors. Slow on vaccines, slow on rapid testing and slow on new drugs, and far too little investment. Still after a year and half of shouting it from the rooftops we are getting some rapid tests. Josh Gans has an interesting reminder focusing on Canada that this has been an example of expert failure not just US failure.
Rapid test advocates such as myself have suddenly moved from fringe crazies who were told they didn’t understand the science to we need them and we need them now.
Several cases in point:
- The CDC now says that unvaccinated students exposed to Covid can “test to stay.” That is, rather than sending all the students in a class (or a school!) home when one tests positive for Covid, they test the students instead and so long as they are negative, they stay.
- The US Government is going to order 500 million rapid tests and distribute them free to the public … by mail!
It is hard to appreciate what a sea change this is in terms of attitude. A year ago, when we tried to roll out rapid tests — that had already been purchased and were sitting in their millions in warehouses in Canada — to Canadian workplaces, we were told that those tests had to be administered by health care professionals in PPE in secure and sanitised environments with all manner of precautions taken that really took the “rapid” out of rapid testing let alone exploding the costs to businesses who wanted to keep their workers safe. This was because they required those long-swabs etc. Eventually, short swabs were permitted. Then self-swabbing supervised in the workplace. Then swabbing at home while on a virtual call with a professional for that supervision with the swabs being picked up and then taken for safe disposal. Finally, we got to self-administered, at-home screening without supervision and you could pop your negative swan in the bin. A year after we had been told that you needed a full-court medical professional press to do this, our kids in Ontario were sent home with 5 rapid tests to use over the holidays. Only a couple of weeks ago, the Ontario government’s advisory board, the Ontario Science Table, finally endorsed the use of rapid tests in this way.
The NYTimes on the FDA and Rapid Tests
In July of 2020 I wrote in Frequent, Fast, and Cheap is Better than Sensitive:
A number of firms have developed cheap, paper-strip tests for coronavirus that report results at-home in about 15 minutes but they have yet to be approved for use by the FDA because the FDA appears to be demanding that all tests reach accuracy levels similar to the PCR test. This is another deadly FDA mistake.
…The PCR tests can discover virus at significantly lower concentration levels than the cheap tests but that extra sensitivity doesn’t matter much in practice. Why not? First, at the lowest levels that the PCR test can detect, the person tested probably isn’t infectious. The cheap test is better at telling whether you are infectious than whether you are infected but the former is what we need to know to open schools and workplaces.
It’s great that other people including the NYTimes are now understanding the problem. Here is the excellent David Leonhardt in Where are the Tests?
Other experts are also criticizing the Biden administration for its failure to expand rapid testing. Even as President Biden has followed a Covid policy much better aligned with scientific evidence than Donald Trump’s, Biden has not broken through some of the bureaucratic rigidity that has hampered the U.S. virus response.
In the case of rapid tests, the F.D.A. has loosened its rules somewhat over the past year, allowing the sale of some antigen tests (which often cost about $12 each). But drugstores, Amazon and other sellers have now largely run out of them. I tried to buy rapid tests this weekend and couldn’t find any.
The F.D.A.’s process for approving rapid tests is “onerous” and “inappropriate,” Daniel Oran and Dr. Eric Topol of Scripps Research wrote in Stat News.
For the most part, the F.D.A. still uses the same cumbersome process for approving Covid tests that it uses for high-tech medical devices. To survive that process, the rapid tests must demonstrate that they are nearly as sensitive as P.C.R. tests, which they are not.
But rapid tests do not need to be so sensitive to be effective, experts point out. P.C.R. tests often identify small amounts of the Covid virus in people who had been infected weeks earlier and are no longer contagious. Rapid tests can miss these cases while still identifying about 98 percent of cases in which a person is infectious, according to Dr. Michael Mina, a Harvard epidemiologist who has been advocating for more testing
Identifying anywhere close to 98 percent of infectious cases would sharply curb Covid’s spread. An analysis in the journal Science Advances found that test frequency matters more for reducing Covid cases than test sensitivity.
As I said on twitter what makes the FDA’s failure to approve more rapid antigen tests especially galling is that some of the tests being sold cheaply in Europe are American tests just ones not approved in the United States. If it’s good enough for the Germans it’s good enough for me!
Update on Rapid Tests for COVID
Nearly a year ago, I wrote Frequent, Fast, and Cheap is Better than Sensitive, arguing for rapid antigen tests:
A number of firms have developed cheap, paper-strip tests for coronavirus that report results at-home in about 15 minutes but they have yet to be approved for use by the FDA because the FDA appears to be demanding that all tests reach accuracy levels similar to the PCR test. This is another deadly FDA mistake.
See also my posts Infected versus Infectious and Rapid Tests. The EMA and then the FDA finally did start approving these tests. So how well are they working? Pretty damn well. Canada has two innovative programs. First, in Nova Scotia pop-up clinics have been using rapid tests for asymptomatic people:
During the third wave that hit Nova Scotia over the past month, the province’s community rapid testing centres have correctly sniffed out at least 285 COVID-19 cases in asymptomatic people, or about 10 per cent of all confirmed cases in this time period, according to the Nova Scotia Health Authority.
While most provinces reserve testing only for symptomatic people or close contacts of a case, Nova Scotia’s pop-up centres allow asymptomatic people to simply show up and get a rapid test for free, with results sent to them within an hour. The whole process relies largely on volunteers without a health-care background.
Furthermore, the true number of cases credited to rapid testing is probably much higher. When a rapid test correctly identifies a positive case, the person’s close contacts such as their family get PCR lab tests that don’t show up in the rapid test statistics.
Lisa Barrett, an infectious diseases specialist and the driving force behind the rapid testing program, said it’s hard to say for certain, but taken altogether it’s possible rapid antigen testing has helped Nova Scotia find up to 18 per cent of all cases during the third wave.
“This is the early detection system,” Barrett said. Rapid testing tends to catch people early on in their infection when they’re full of virus, meaning positive cases are found and put into isolation fast — likely days before they would have been found with a PCR test, if they were found at all.
Michael Mina argues that since the rapid antigen detected cases are among the most infectious cases, detecting these cases is probably worth half of all the PCR testing.
Second, Canada’s CDL Rapid Screening Consortium is now in 200 sites with 50 large companies and rapidly expanding. A very interesting, just published paper in The Lancet runs an experiment that suggests that these testing regimes can work. The experiment rapidly tested 1000 people and the negatives were then randomly assigned either to be sent-home to conduct their regular life or to attend a multi-hour concert with masks but also singing, dancing, alcohol and no-social distancing. After 8 days there were two infections in the at-home group and no infections in the Concert group which suggests that this type of rapid testing can be used to open and keep-open concerts, schools, universities, airplanes and workplaces.
What’s the point of testing now that we have vaccines? Two reasons. First, most of the world still hasn’t been vaccinated so testing will be a very useful stop-gap measure until vaccination is more widely distributed. Indeed, the success of these programs shows what we lost by not acting more quickly a year ago. Second, although the pandemic is (essentially) over in the United States (as predicted) there will likely be an uptick in the fall among the unvaccinated and you want rapid tests to be available rapidly in hot-spots. In other words, rapid deployment of rapid tests will help us to avoid outbreaks in the future.
The problem with rapid testing was always on the demand side
The U.S. government distributed millions of fast-acting tests for diagnosing coronavirus infections at the end of last year to help tamp down outbreaks in nursing homes and prisons and allow schools to reopen.
But some states haven’t used many of the tests, due to logistical hurdles and accuracy concerns, squandering a valuable tool for managing the pandemic. The first batches, shipped to states in September, are approaching their six-month expiration dates.
At least 32 million of the 142 million BinaxNOW rapid Covid-19 tests distributed by the U.S. government to states starting last year weren’t used as of early February, according to a Wall Street Journal review of their inventories…
“The demand has just not been there,” said Myra Kunas, Minnesota’s interim public health lab director.
…the tests are piling up in many states, the Journal found.
Here is more from Brianna Abbott and Sarah Krouse at the WSJ. You may recall the discussions of demand-side issues from my CWTs with Paul Romer and Glen Weyl. The envelope theorem remains underrated.
Rapid Tests
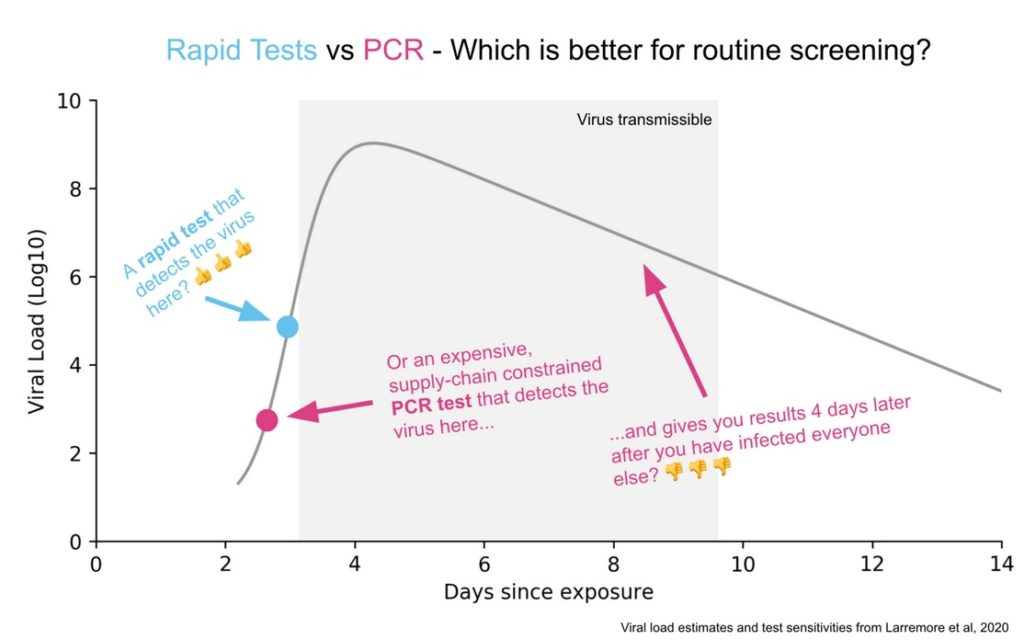
Here’s a good picture illustrating the difference between the PCR and Rapid Test. A PCR amplifies DNA and so if taken at the right time it will detect the virus before a rapid test will. But this happens when there isn’t much viral load and too little of the virus to be transmissible. Moreover, at these times, the virus is increasing rapidly so the rapid test will find the virus tomorrow. The PCR test will also pick up fragments after transmissiblity has passed which also isn’t very useful. A rapid test is very sensitive for doing what it is supposed to do, identifying periods of infectiousness.
Michael Mina has done a great job promoting rapid tests and I do think we are beginning to see some recognition of the difference between infected versus infectious and the importance of testing for the latter. What is frustrating is how long it has taken to get this point across. Paul Romer made all the key points in March! (Tyler and myself have also been pushing this view for a long time).
In particular, back in March, Paul showed that frequent was much more important than sensitive and he was calling for millions of tests a day. At the time, he was discounted for supposedly not focusing enough on false negatives, even though he showed that false negatives don’t matter very much for infection control. People also claimed that millions of tests a day was impossible (Reagents!, Swabs!, Bottlenecks!) and they weren’t impressed when Paul responded ‘throw some soft drink money at the problem and the market will solve it!’. Paul, however, has turned out be correct. We don’t have these tests yet but it is now clear that there is no technological or economic barrier to millions of tests a day.
Go yell at your member of Congress.
Retrospective look at rapid Covid testing
To be clear, I still favor rapid Covid tests, and I believe we were intolerably slow to get these underway. The benefits far exceed the costs, and did earlier on in the pandemic as well.
That said, with a number of pandemic retrospectives underway, here is part of mine. I don’t think the strong case for those tests came close to panning out.
I had raised some initial doubts in my podcasts with Paul Romer and also with Glen Weyl, mostly about the risk of an inadequate demand to take such tests. I believe that such doubts have been validated.
Ideally what you want asymptomatic people in high-risk positions taking the tests on a frequent basis, and, if they become Covid-positive, learning they are infectious before symptoms set in (remember when the FDA basically shut down Curative for giving tests to the asymptomatic? Criminal). And then isolating themselves. We had some of that. But far more often I witnessed:
1. People with symptoms taking the tests to confirm they had Covid. Nothing wrong with that, but it leads to a minimal gain, since in so many cases it was pretty clear without the test.
2. Various institutions requiring tests for meet-ups and the like. These tests would catch some but not all cases, and the event still would turn into a spreader event, albeit at a probably lower level than otherwise would have been the case.
3. Nervous Nellies taking the test in low-risk situations mainly to reassure themselves and others. Again, no problems there but not the highest value use either.
So the prospects for mass rapid testing — done in the most efficacious manner — were I think overrated.
I recall the summer of 2022 in Ireland, which by the way is when I caught Covid (I was fine, though decided to spend an extra week in Ireland rather than risk infecting my plane mates). Rapid tests were available everywhere, and at much lower prices than in the United States. Better than not! But what really seemed to make the difference was vaccines. The availability of all those tests did not do so much to prevent Covid from spreading like a wildfire during that Irish summer. Fortunately, deaths rose but did not skyrocket.
The well-known Society for Healthcare Epidemiology just recommended that hospitals stop testing asymptomatic patients for Covid. You may or may not agree, but that is a sign of how much status testing has lost.
Some commentators argue there are more false negatives on the rapid tests with Omicron than with earlier strains. I haven’t seen proof of this claim, but it is itself noteworthy that we still are not sure how good the tests are currently. That too reflects a lower status for testing.
Again, on a cost-benefit basis I’m all for such testing! But I’ve been lowering my estimate of its efficacy.
Update on Rapid Antigen Tests
In July of 2020 in Frequent, Fast and Cheap is Better than Sensitive, I wrote:
A number of firms have developed cheap, paper-strip tests for coronavirus that report results at-home in about 15 minutes but they have yet to be approved for use by the FDA because the FDA appears to be demanding that all tests reach accuracy levels similar to the PCR test. This is another deadly FDA mistake.
It’s depressing that we are still moving so slowly on these issues but the media has finally gotten on board. Earlier I mentioned David Leonhardt’s article. Here is Margaret Hartmann in the New York Magazine.
In many Asian and European countries, at-home COVID-19 tests are cheap and easy to find in stores. CBS News reported this month that home antigen tests are now used routinely in the U.K., where they are free and “readily available at pretty much every pharmacy in the country.”
The situation is drastically different here because U.S. health officials focused on getting people vaccinated against COVID-19 and never leaned into asymptomatic testing as a strategy to fight the pandemic. While some foreign governments moved quickly to encourage screening and subsidize the cost of at-home tests, the Food and Drug Administration’s approval process moved much more slowly.
….The FDA said it needed to ensure that the tests were accurate, but many scientists countered that the agency was letting the perfect be the enemy of the good.
Note also that this is a way of saying that the politicians have now also had it with the FDA:
In addition to ramping up production of tests already on the market, the government is also working to speed up the approval process. On October 4, the FDA authorized Flowflex, an at-home antigen test produced by ACON Laboratories that is expected to retail for around $10 per test. And on October 25, the Department of Health and Human Services announced that the FDA will streamline its authorization process, and the National Institutes of Health will spend $70 million on a new program to “establish an accelerated pathway” to aid test makers seeking approval for their products.
FDA Approves American Rapid Antigen Test
What makes the FDAs failure to approve more rapid antigen tests even more galling is that the test being sold cheaply in the Amsterdam supermarket is the Flowflex, an American test made by Acon Labs in San Diego.
Well the FDA has finally approved the Acon test! Apparently it is good enough for the Germans and for US citizens. Hoorah! USA Today notes:
ACON expects to make 100 million tests per month by the end of this year. Production could double to 200 million monthly tests by February, according to the FDA.
…The United Kingdom and Germany have made significant purchases of home tests and widely distributed them to their residents to slow the spread of coronavirus. Such large government purchases allowed manufacturers to continue making tests even when demand softened as cases dropped.
The Biden administration will spend nearly $1.2 billion to purchase up to 187 million home tests from Abbott Laboratories and Celltrion Inc., company officials confirmed. The Department of Defense announced additional contracts totaling $647 million to buy 60 million kits from Abbott and three other testing vendors: OraSure Technologies, Quidel and Intrivio Holdings.
The FDA has authorized seven antigen-based tests that can be used at home without a prescription. The EU has authorized 21 tests beginning with the letter A (I am not sure all of these are authorized for home use but you get the idea.) Turtle slow. Still this is a big improvement.
Frankly, I think all the pressure from people like Michael Mina amplified by myself and others over 18 months and culminating in David Leonhartd’s NYTimes article Where Are the Tests? finally pushed them over the edge.
Rapid Antigen Tests in Canada
Josh Gans announces a program of Rapid Antigen Tests in Canada backed by a consortium of major Canadian companies.
Big News! Today I am very pleased to be able to reveal to the world something that I have been very proud to have been working on with a hundred or so other people: The CDL Rapid Screening Consortium. Led by our Creative Destruction Lab, this consortium is a group of 12 companies who are partnering with Health Canada to begin the roll-out of rapid antigen screens to be a part of daily life for the next 12-18 months and deliver a safer path to normality. We have been working since September intensively to put the consortium together, explore screening options that were available globally and come up with protocols and an evolving standard operating procedure (SOP) to bring rapid antigen screens at scale to economies all around the world. The goal is to solve the pandemic information gap and ensure that we can quickly identify and isolate infectious people and protect others.
The initial sites will be run by RSC members. Those members are Air Canada, Rogers, Loblaws, Shoppers Drug Mart, Magna, Nutrien, Suncor, Genpact, Scotiabank, MDA, CPPIB and MLSE.
Read Josh’s announcement for more details and here is the website for the CDL Rapid Screening Consortium.
Rapid Antigen Tests in Europe
Why are these tests important? The CDC now says that asymptomatic or pre-symptomatic people account for a majority of infections. Do you get it? How many people without symptoms will get a COVID PCR test, which can be time consuming and expensive? (And how many PCR tests can we run in a timely fashion if people without symptoms get many more tests?) Not that many. But many people without symptoms would get a $8 or less, at-home, 15 minute test. And if some of those people discover that they are infectious and self-isolate for a few days we can drive infection rates down.
We should have had an Operation Warp Speed for tests. We still need funding for a mass rollout and, of course, the FDA needs to approve these tests! (Here is Michael Mina in Time fulminating at the FDA holdup.)
By the way, more than 2800 Americans have died of COVID since Pfizer requested an Emergency Use Authorization for their vaccine. The FDA meets Dec. 10.
Addendum: Here’s me explaining why Frequent, Fast, and Cheap is Better than Sensitive and the difference between infected and infectious.
Rapid Antigen Tests
Rapid antigen tests are starting to be adopted worldwide.
Reuters: Germany, where infections jumped by 4,122 on Tuesday to 329,453 total, has secured 9 million so-called antigen tests per month that can deliver a result in minutes and cost about 5 euros ($5.90) each. That would, in theory, cover more than 10% of the population.
The United States and Canada are also buying millions of tests, as is Italy, whose recent tender for 5 million tests attracted offers from 35 companies.
Germany’s Robert Koch Institute (RKI) now recommends antigen tests to complement existing molecular PCR tests, which have become the standard for assessing active infections but which have also suffered shortages as the pandemic overwhelmed laboratories and outstripped manufacturers’ production capacity.
See my earlier posts Frequent, Fast, and Cheap is Better than Sensitive, Infected versus Infectious, and Rapid Tests for more on these types of tests.
The problem with rapid Covid testing
Mayank Gupta emails me:
The absolute number of false positives would rise dramatically under slightly inaccurate, broad surveillance testing. At least initially, the number of people going to the doctor to ask what to do would also rise. One can imagine if doctors truly flubbed and didn’t know how to advise patients accurately, a lot of individual patients would lose trust in the medical system (testing, doctors, or both). The consequence of this would be more resistance to health public policy measures in the future.
I see this as quite similar to what happened on three mile island. There was a clear utilitarian benefit to taking on some small amount of nuclear accident risk. The public was never taught how or why to internalize and cope with this risk. When the risk manifested, individuals saw the risk but not the public benefit and turned against nuclear. This change of public opinion was reflected in public policy after.
I’m sure there’s some mismatches in this analogy, but I’m using it to point out that in general I find thinking on the margin without thinking about the public’s ability to think on the margin can result in setbacks on the margin.
In an age where science is both more capable of solving social problems and more complex to understand, but the public has too much complex information to sort through, the central problem of governance seems to be how to solve public choice, without creating a monopoly on information.
To be clear, I do favor rapid testing, but it is worth giving this problem further thought.
Testing Freedom
In the latest Discourse Magazine I discuss the FDA’s long-standing fear and antipathy toward personalized medical tests and how this violates the 1st Amendment.
In 1972, the FDA confiscated thousands of home pregnancy tests, declaring that they were “drugs” meant to diagnose a “disease” and thus fell under the FDA’s regulatory dominion. The case went to the U.S. District Court for the District of New Jersey, and Judge Vincent P. Biunno ruled that the FDA had overstepped. “Pregnancy,” he said, “is a normal physiological function of all mammals and cannot be considered a disease … a test for pregnancy, then, is not a test for the diagnosis of disease. It is no more than a test for news….” As a result of Judge Biunno’s ruling, home pregnancy tests are easily available today from pharmacies, grocery stores and online shops without a prescription.
These days, debates over home pregnancy tests from the 1970s seem anachronistic and paternalistic. Yet the same paternalistic arguments appear again and again with every new testing technology. In the late 1980s, for example, the FDA simply declared that it would not approve at-home HIV tests, regardless of their safety or efficacy. As with pregnancy tests, the concern was that people could not be trusted with information about their own bodies…the first rapid at-home HIV test was developed and submitted to the FDA in 1987 [but] it took 25 years before the FDA would approve these tests. (Now, you can easily buy such a test on Amazon.)
…The FDA has a vital role in ensuring that tests are clinically accurate—tests should do what they say they do. Tests don’t need to be perfectly accurate to be useful (think of thermometers, personality tests and tire pressure gauges), but if a test advertises that it measures HDL cholesterol, it should do that within the tolerances the firm promises. The FDA has the technical knowledge to ensure that tests work, and that’s a skill that Americans value from the agency.
What Americans don’t want is to be told they can’t handle the truth. Yet when it came to at-home tests such as pregnancy tests, HIV tests and genetic tests, that’s exactly the reasoning the FDA used—and continues to use—to suppress information. The FDA should ensure that tests are safe, but “safety” means physical safety. The FDA may not declare a product unsafe because it might produce dangerous knowledge. Patients have a right to know about their own bodies. Our antibodies, ourselves. The FDA has authority over drugs and devices but not over patients.
Judge Biunno had it right back in 1972 when he said that diagnostic tests produce “news.” Test results, therefore, are a type of speech that fall under the First Amendment right to freedom of speech. The Supreme Court has repeatedly rejected restrictions on freedom of speech based on “a fear that people would make bad decisions if given truthful information”; thus, FDA restrictions on tests based on such fears are unconstitutional. The question of whether consumers will respond “safely” to test results is no more relevant to the FDA’s regulatory authority than the question of whether readers will respond safely to political news published in The New York Times. The FDA does not have the constitutional authority to regulate news.