San Diego biotech company making melanoma test patch cuts 56% of its workforce
San Diego-based DermTech is also exploring the possibility of a merger, acquisition, sale of assets, licensing or other transaction.
DermTech, the San Diego-based company that developed a noninvasive skin sticker to detect melanoma, is cutting its workforce in half and exploring a possible sale.
The local biotech announced Thursday that it is restructuring “to significantly reduce expenses associated with its current operations to preserve cash.” This includes reducing its headcount by 56 percent, or about 100 employees.
DermTech debuted on the public market in 2019 through a reverse merger. As of Dec. 31, 2023, it employed 208 people; however, the company laid off about 30 workers earlier this year.
The company’s layoff of 101 employees takes effect June 17, according to the Worker Adjustment and Retraining Notifications (WARN) letter filed with the state. The workforce reductions includes regional sales managers, directors, vice presidents as well as DermTech’s chief compliance and chief information officers.
The San Diego biotechnology company also laid off its chief operating officer as it continues to cut costs and streamline operations
DermTech did not disclose the expected cost savings from this layoff. The restructuring will cost the company a one-time charge of approximately $1.6 million during the second quarter of 2024, according to its filing with the Securities and Exchange Commission.
The company’s board of directors is also working with investment banking firm TD Cowen to examine the options available, including “an acquisition, merger, reverse merger, business combination, sale of assets, licensing or other transaction involving” DermTech.
As a result of this news, DermTech will not hold a first-quarter earnings call with investors.
DermTech said it “intends to continue laboratory operations during the strategic review and believes it has sufficient capacity to process orders for the DermTech Melanoma Test.”
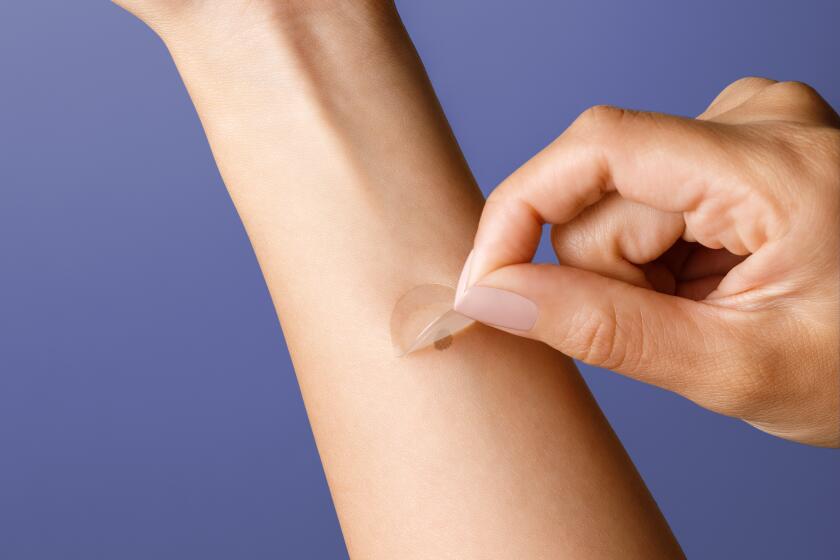
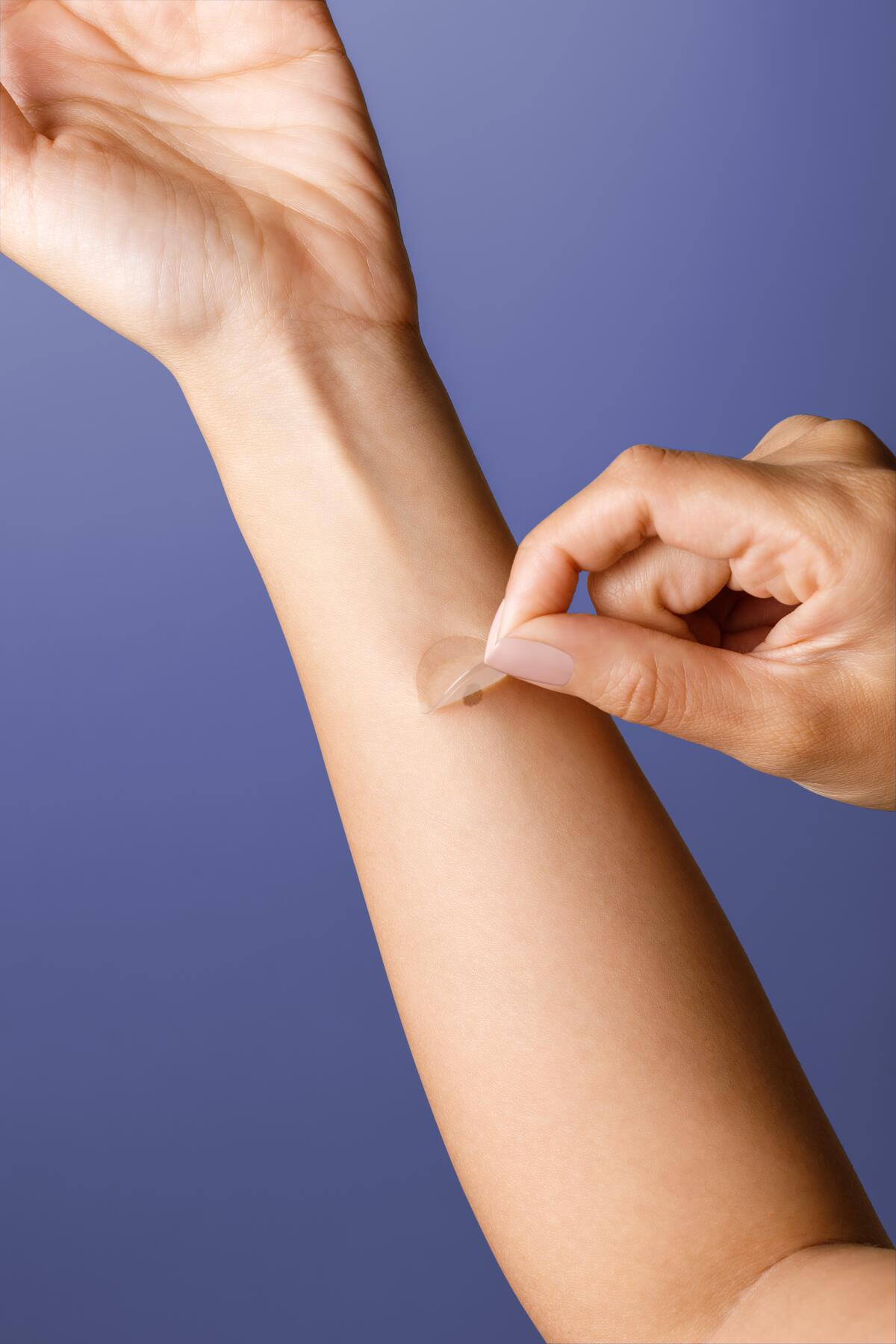
The novel, noninvasive genomic approach to detecting skin cancer relies on a smart sticker that is placed on a mole or dark skin patch. Rather than using a scalpel to collect skin tissue, the sophisticated sticker gathers samples from sensitive areas such as the neck, face and chest.
The samples are then sent to a certified laboratory to process the results.
The U.S. Food and Drug Administration has not reviewed or approved the commercial test. However, DermTech’s melanoma test has gained insurance coverage with many groups, including Medicare, Veterans Affairs and certain commercial insurers.
Biotech firm also suspends research on certain programs to focus resources on its non-invasive, genomics based-test for skin cancer
The tests are marketed to dermatologists, especially where DermTech has achieved coverage. According to the company’s most recent annual report filed in February, “more than 130 million patients have reimbursable access” to this test.
DermTech has been trying to stabilize its financial situation over the past year. This marks the third restructuring since June, when the company cut jobs and shelved certain programs after a change in leadership.
In February, the company reduced its headcount by 15 percent to cut costs.
In 2023, the company recorded revenue of $15.2 million — a 5 percent increase from the previous year. A majority of this revenue came from the melanoma test. DermTech reported a net loss of $100.9 million in 2023, an improvement from the $116.7 million loss recorded in 2022.
This week, the company also received a notice from the Nasdaq saying its stock does not meet requirements to continue trading, according to an SEC filing. DermTech’s stock has been trading below $1 per share for 30 consecutive days.
The company has until Oct. 14 to regain compliance to continue trading on the Nasdaq.
Updates
6:22 p.m. April 25, 2024: This story was updated with details from DermTech’s Worker Adjustment and Retraining Notifications (WARN) letter filed with the state.
Get U-T Business in your inbox on Mondays
Get ready for your week with the week’s top business stories from San Diego and California, in your inbox Monday mornings.
You may occasionally receive promotional content from the San Diego Union-Tribune.