-
PDF
- Split View
-
Views
-
Cite
Cite
Ulrich Sonnenborn, Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties, FEMS Microbiology Letters, Volume 363, Issue 19, October 2016, fnw212, https://doi.org/10.1093/femsle/fnw212
Close - Share Icon Share
Among the gram-negative microorganisms with probiotic properties, Escherichia coli strain Nissle 1917 (briefly EcN) is probably the most intensively investigated bacterial strain today. Since nearly 100 years, the EcN strain is used as the active pharmaceutical ingredient in a licensed medicinal product that is distributed in Germany and several other countries. Over the last few decades, novel probiotic activities have been detected, which taken together are specific of this versatile E. coli strain. This review gives a short overview on the discovery and history of the EcN strain.
INTRODUCTION: MEDICAL-HISTORICAL BACKGROUND
In the second half of the 19th century, microbiology parted with botany and quickly established itself as a new independent scientific discipline. Microbiological research in general, and especially research in medical microbiology, saw an enormous boost. The time from about 1861 (Pasteur disproved spontaneous generation of life) till 1917 (d'Hérelle coined the term ‘bacteriophages’ for viruses that infect bacteria) is thus called ‘the first golden age of microbiology’ (Maloy and Schaechter 2006). The scientific upturn of medical microbiology was first of all a ‘golden age’ of bacteriology (Blevinsz and Bronze 2010). During this period, many different bacterial species were identified as etiologic agents of diverse human and animal infectious diseases. The increased significance of microbiology was a result of the ground-breaking discoveries of Louis Pasteur, Robert Koch, Joseph Lister, Albert Neisser, Friedrich Loeffler, Karl Joseph Eberth, David Bruce, Alexandre Yersin, Shibasaburo Kitasato and Paul Ehrlich, to name just a few.
At the same time, other pioneer microbiologists and immunologists, as for instance Theodor Escherich and Elie (Ilja) Metchnikoff, were interested in host–microbiota interactions and their relations to human health and disease (Escherich 1886; Metchnikoff 1907). One of the controversially discussed topics at that time was the question, whether the so-called normal microflora of the human gut or specific intestinal microorganisms might be more beneficial or more harmful to the host. Another unsolved question was, how the coexistence of the bacteria in the gut might be regulated and what kind of bacteria–bacteria interactions and bacteria–host interactions are taking place.
The latter was a topic that also interested Alfred Nissle (Fig. 1), working since 1912 as first medical assistant in hygiene and bacteriology at the Hygiene Institute of the Albert-Ludwigs-University of Freiburg, Germany. From 1915 till 1938, Nissle was also head of the ‘Baden Medicinal Investigations Office for Infectious Diseases’ in Southwest Germany, which is still affiliated with the university's Hygiene Institute to this day. This position gave him considerable leeway to follow his research interests, but eventually led to conflicts with his supervisor Hermann Dold, tenured professor of the Hygiene Institute. In 1938, Nissle left the Hygiene Institute and established his own private laboratory in Freiburg that he ran till his death in 1965.
Portrait photo of Prof. Dr med. Alfred Nissle (30 December 1874 to 25 November 1965). Nissle was the discoverer of bacterial antagonism of certain intestinal E. coli strains against pathogenic enterobacteria. In 1917, he isolated the ‘antagonistically strong’ E. coli strain, which is now named for him: E. coli Nissle 1917 (EcN). He developed the live biotherapeutic product Mutaflorthat contains E. coli strain Nissle 1917 as the active principle. (Photo taken from Irrgang and Sonnenborn 1988, by courtesy of Ardeypharm GmbH.)
THE DISCOVERY OF ESCHERICHIA COLI ANTAGONISM
In bacteriological practical courses, when medical students were encouraged to grow Petri-dish cultures with stool samples, spiked with pure cultures of pathogenic Salmonella strains, Nissle had observed that in most cases the Salmonella colonies clearly grew out of the lawn of the other bacteria. However, in rare cases, the Salmonellae grew poorly or even not at all. Instead, Escherichia coli colonies dominated. This led Nissle to the idea that in such cases the respective stool samples might contain E. coli strains that were able to impede the growth of the added Salmonella strains. Later, Nissle could show in laboratory experiments that in fact certain isolates of E. coli from the intestinal microbiota of healthy humans were able to inhibit the growth of co-cultured Salmonella strains and other coliform enteropathogens (Nissle 1916). For this special bacterial feature, Nissle coined the term ‘antagonistic activity’. For the assessment of the strength of antagonistic activity, Nissle developed an ‘antagonistic index’ based on quantitative in vitro co-culture assays in liquid medium (‘antagonism test’) and was thus able to differentiate between ‘antagonistically strong’ and ‘antagonistically weak’ E. coli strains.
However, Nissle did not further investigate which biochemical substances were responsible for these antagonistic effects. Being not only a bacteriologist, but still more a medical doctor working in the pre-antibiotic era, Nissle was more interested in investigating whether this principle of bacterial interference might be useful to treat gastrointestinal disturbances and diseases caused by pathogenic bacteria (Loew 2001). On 20 June 1916, Nissle presented his results on bacterial antagonism and his thoughts on its medical applicability in a lecture in front of the Freiburg Medical Association entitled ‘Ueber die Grundlagen einer neuen ursächlichen Bekämpfung der pathologischen Darmflora’ (On the fundamentals for a novel causal control of pathological gut flora). This lecture was published in the Deutsche Medizinische Wochenschrift (Nissle 1916) (Fig. 2) and was the starting point for a large number of medical publications on case studies and case digests by Nissle and other physicians, mainly from German-speaking countries in Central Europe, as to how antagonistically active E. coli strains can be applied in prophylaxis and therapy (e.g. Nissle 1918; Geisse 1919; Rörig 1921; Werlé 1921; Nissle 1925; Ulrich 1926; Nissle 1948). More recent randomized controlled clinical trials proved the validity of Nissle's concept of applying E. coli antagonism to prevent or treat diarrhoea (e.g. Lodinova-Zadnikova and Sonnenborn 1997; von Bünau et al. 2005; Henker et al. 2007).
Title page of Nissle's first publication on E. coli antagonism in the Deutsche Medizinische Wochenschrift, September 1916, in which he described the antagonistic activity of certain intestinal E. coli strains, its determination and possible medical applicability. (Photo taken from Irrgang and Sonnenborn 1988, by courtesy of Ardeypharm GmbH.)
With the advent of the antibiotic era, the interest of the medical community in the treatment option of combating intestinal diseases with living antagonistically active bacteria strongly declined. Only in the last 20 years, when the increase of antibiotic resistance became a world-wide problem (Hawkey 1998), interest in alternative microbiology-based methods for fighting infectious and inflammatory bowel diseases regrew (Sartor 2005; Fric 2007). The renaissance of this kind of ‘Microbiological Therapy’ with living beneficial microorganisms in the new millennium was also stimulated by the intensification of basic research in intestinal microbiology and microecology employing novel methods of molecular biology and bioinformatics (e.g. in the ‘Human Microbiome Project’) (The Human Microbiome Project Consortium 2012).
ORIGIN OF ESCHERICHIA COLI STRAIN NISSLE 1917
The Escherichia coli strain nissle 1917 (EcN) strain was isolated by Alfred Nissle in 1917 during the First World War from the faeces of a German soldier. This soldier was a corporal of the armed forces, being at that time in a field hospital near Freiburg, who had joined the military campaign on the Balkans before. Deployed in the region of Dobrudja for some time, which was then heavily contaminated by Shigella, this soldier, in contrast to his comrades, did not develop diarrhoea or any other intestinal disease (Nissle 1925). Nissle suspected this soldier to carry an antagonistically strong E. coli strain in his gut that might have protected him from catching diarrhoea. As suspected, Nissle could isolate from a stool sample of this soldier an E. coli strain that in laboratory tests showed strong antagonistic activity against different pathogenic enterobacteria (Nissle 1925).
ESCHERICHIA COLI NISSLE 1917: 100 YEARS OF MEDICAL APPLICATION
As described above, Nissle isolated the particularly high-grade antagonistically active E. coli strain from the German corporal in 1917. This strain of serotype O6:K5:H1 was later named after his discoverer ‘E. coli strain Nissle 1917 (EcN)’. Nissle deposited this E. coli isolate in his laboratory culture collection and for the treatment of individual patients first cultured the strain on large agar plates in his laboratory, filled the bacteria in gelatine capsules and sealed them with wax or with paraffin. In 1917, Nissle obtained a patent for the Mutaflor trademark by the then existing Imperial Patents Office in Berlin. The name Mutaflor, deduced from the Latin words mutare and flora, was meant to express the capability of the EcN strain to change the intestinal microflora by its antagonistic activity.
To produce adequate quantities of this biotherapeutic agent in order to treat more patients, Nissle first gave the EcN strain to the pharmaceutical company G. Pohl in Schoenbaum near Danzig (today Gdansk, Poland), since they had special experience in the production of gelatine capsules. This company undertook manufacturing and sales of the Mutaflor preparation till 1932, when Nissle transferred the licence for production to Hageda AG in Berlin, because of commonly occurring supply difficulties. These were caused by the fact that after the First World War the city of Danzig was partitioned off the German Reich and became the status of a ‘free state’, now isolated from the main part of Germany by the so-called Polish Corridor. In December 1944, the manufacturing facilities in Berlin were destroyed by a bomb raid of the Allied Forces. After the war, Hageda AG reconstructed their manufacturing facilities in Hannover and resumed Mutaflor production. All the time Nissle provided subcultures of the EcN strain as starter cultures for each production batch of the Mutaflor preparation till his death in November 1965 (Irrgang and Sonnenborn 1988; Loew 2001).
After Nissle's death, all rights pertinent to Mutaflor were assigned to Hageda AG. In 1971, the company relinquished their production facilities in Hannover and sold on the rights to Ardeypharm GmbH of Herdecke/Germany in February 1971 (Irrgang and Sonnenborn 1988; Loew 2001). Later, the EcN strain was deposited by Ardeypharm in the collection of industrially used bacterial strains at the German Collection for Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen, DSMZ), where it got the designation ‘E. coli DSM 6601’.
At the beginning of the 1980s, Ardeypharm started a cooperation with the Society for Biotechnological Research (Gesellschaft für Biotechnologische Forschung, GBF) in Braunschweig/Germany in order to modernize the production by introducing fermentation technology at the manufacturing location in Herdecke. This was finally established in 1984. Since that time state-of-the-art fermentation technology has been used for culturing of large amounts of EcN, nowadays as part of a standardized production according to Good Manufacturing Practice (GMP).
MODERN BIOMEDICAL RESEARCH ON ESCHERICHIA COLI NISSLE 1917—NEW INSIGHTS INTO PROBIOTIC MODES OF ACTION
In the mid-1980s, a comprehensive research programme was started by Ardeypharm to thoroughly characterize the EcN strain, phenotypically as well as genetically, and to reveal the peculiarities of this special E. coli strain by analysing its microbiological, biochemical, pharmacological, immunological and toxicological characteristics and activities. Since it had been shown that different E. coli varieties acted as intestinal or extraintestinal pathogens, this raised the question of whether the EcN strain might have any pathogenic properties that could not have been detected by the methods available during the Nissle era. Thus, biosafety aspects of the EcN strain have been one of the primary research topics during the past decades (Sonnenborn & Schulze 2009). In summary, none of the pathogenic properties known of enteropathogenic or extraintestinally pathogenic E. coli have been found. This finding was substantiated by molecular genetic analyses of the EcN strain, including the elucidation of its complete plasmidic (Blum-Oehler et al. 2003) and genomic DNA sequences (Reister et al. 2014). Further special characteristics and pleiotropic activities of the EcN strain discovered in the last 30 years by us and by a large number of cooperating working groups world-wide have been the subject of several comprehensive reviews (e.g. Sonnenborn and Schulze 2009; Oelschlaeger 2010; Behnsen et al. 2013; Sassone-Corsi and Raffatellu 2015; Scaldaferri et al. 2016). Therefore, these features of the EcN strain will only be briefly summarized here.
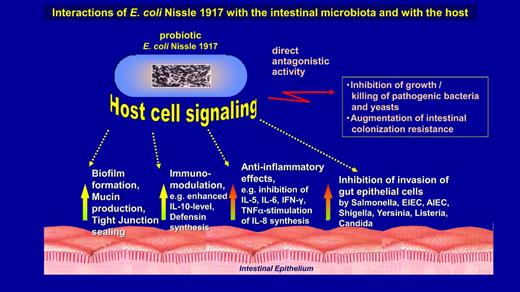
As explained above, Alfred Nissle used the EcN strain for the treatment of patients because of its strong antagonistic activity against closely related enteropathogens. This kind of antimicrobial activity may be called a direct antagonistic effect (Fig. 3, top right).
Interactions of E. coli strain Nissle 1917 with the intestinal microbiota and with the host. Direct antagonistic effects against pathogens (top right) were first described by Alfred Nissle. Inhibition of invasion of gut epithelial cells by enteroinvasive pathogens are indirect antagonistic effects (at the bottom on the right) and involve signaling with the gut mucosa. All the other new probiotic activities that have been discovered in the last few decades involve communication with host tissues, e.g. with the gut mucosa and/or the gut immune system.
The molecular background of this direct antagonism was addressed for the first time by Gratia (1925) and later accomplished by many others. The antagonistically active molecules, synthesized by different E. coli strains were identified as high-molecular mass proteins (>10 000 Dalton), termed colicins (Fredericq 1957). Colicin producers primarily affected other susceptible near relatives, i.e. other Enterobacteriaceae. Later on, Asensio, Baquero and colleagues discovered another class of low-molecular mass peptides (<10 000 Dalton) produced by some intestinal E. coli strains that inhibited the growth of other sensitive enterobacteria (Baquero and Moreno 1984). These antibacterial peptides were named microcins. It has been speculated that the microcins – because of being protease resistant – are of ecological importance for inter-bacterial interactions in the gut (De Lorenzo and Aguilar 1984).
Some decades later, it has been shown that the direct antagonistic activity of EcN was partly due to the production of two microcins (microcin H47 and microcin M [‘M’ for Mutaflor]) (Patzer et al. 2003). Another peculiarity that helps the EcN strain to antagonize near relatives, such as Salmonella typhimurium, and to establish itself in the gut is the ability to produce a broad range of siderophores and other iron acquisition systems (Deriu et al. 2013).
Besides the direct antagonistic action against other microbes in vitro and in vivo, EcN exhibits an indirect antagonistic action against enteroinvasive bacteria involving signalling to the host epithelium (Fig. 3, on the bottom right) (Altenhoefer et al. 2004). As summarized in Fig. 3, the EcN strain moreover interacts in many ways with the intestinal epithelium and the mucosal immune system, stimulating e.g. non-specific defence mechanisms, such as the synthesis of human β-defensin-2 in colonic epithelial cells (Wehkamp et al. 2004). It also shows immunomodulatory and anti-inflammatory activities, and has a restorative effect on the epithelial permeability barrier, thereby preventing the development of ‘leaky gut’ symptoms in cases of a disturbed barrier function (Ukena et al. 2007; Zyrek et al. 2007).
CONCLUSION AND OUTLOOK
In the food sector, the term ‘probiotics’ stands for products containing live non-pathogenic microorganisms that confer a health benefit on the host, when administered in adequate amounts (FAO/WHO 2006). In the medico-pharmaceutical sector, the preferred corresponding official term for this kind of products is ‘Live Biotherapeutic Products’ (EDQM 2015), although in the pharmaceutical, medical and microbiological literature the term ‘probiotic’ is also commonly used. In the food industry, probiotic products mainly contain lactic acid bacteria, such as lactobacilli, lactococci and bifidobacteria. These probiotics are not intended for the treatment of diseased human beings, but are thought to retain health and well-being and to reduce the long-term risk of developing diverse diseases in otherwise healthy people. In pharmaceutical products used in human and veterinary medicine, the intended use is another one and also non-pathogenic microorganisms, e.g. certain yeast strains (Saccharomyces boulardii) or Escherichia coli strains (E. coli Nissle 1917) are applied in prophylaxis and therapy since several decades.
Today, EcN is probably the most intensively investigated Escherichia coli strain used in a probiotic preparation. For 100 years, this strain has been and still is the active principle of a licensed medicinal product (Mutaflor), which is distributed on the pharmaceutical market in Germany and several other countries (Schultz 2008; Sonnenborn and Schulze 2009; Jacobi and Malfertheiner 2011; Behnsen et al. 2013; Scaldaferri et al. 2016). As stated by Jacobi and Malfertheiner (2011), intensive research on the EcN strain during the last few decades has resulted in getting ‘new insights into an old probiotic bacterium’. Quite a lot of hitherto unknown probiotic activities have been detected that taken together are specific of this versatile E. coli strain (Sonnenborn and Schulze 2009; Oelschlaeger 2010; Behnsen et al. 2013).
As a consequence, E. coli Nissle 1917 is nowadays often used as a reference strain or model microorganism in experimental biomedical studies (Sonnenborn and Schulze 2009). Moreover, ongoing studies on recombinant manipulations of the EcN genome open the chance to construct EcN derivatives with novel properties, which in the future might be useful as carriers of bioactive molecules or vector systems for live oral vaccine development.
The assistance in obtaining the relevant literature by Martina Schiemann, Herdecke, is gratefully acknowledged. The author is much obliged to Ardeypharm GmbH for supplying the photographs of Alfred Nissle and of the title page of Nissle's publication in 1916 on E. coli antagonism.
DECLARATION OF INTEREST
The author was head of the Biological Research Division of Ardeypharm GmbH, Herdecke, until his retirement in April 2016. The company produces and distributes the licensed live biotherapeutic product Mutaflor, containing EcN as the active pharmaceutical ingredient. The company had no influence on the content of the manuscript. The author alone is responsible for the content and writing of this paper.
Conflict of interest. None declared.
REFERENCES