This blog was written by Mark Manfredi, CEO of Kyn Therapeutics and former Atlas Entrepreneur-in-Residence, as part of the From The Trenches feature of LifeSciVC.
Last week we learned about the failure of the much anticipated ECHO-301/KEYNOTE-252 trial that studied the IDO inhibitor epacadostat in combination with pembrolizumab in advanced melanoma. The results were a surprise to some, anticipated by others, and either way a bad outcome for IDO1 inhibitors, Incyte, and others heavily invested in IDO inhibition as a therapeutic approach. More significantly, it was a disappointment for cancer patients and their families looking for the next generation of immunotherapies to improve patient outcomes.
ECHO-301 was designed as a follow-on confirmatory phase 3 study of a phase 1/2 single arm combination trial which demonstrated encouraging overall response rates and a progression free survival (PFS) superior to pembrolizumab alone in an equivalent patient population (based on historical data). Despite the lack of efficacy, there is much to be gleaned from this failed study now and as further information is revealed. I believe the broad field of immuno-metabolism will ultimately benefit from lessons learned. Many have written about the specifics of the trial design; so rather, I will focus my initial reactions on why I remain bullish on targeting IDO1/TDO biology as a way to boost immune responses in tumors.
For those who don’t want to be bludgeoned by the details, here are quick takeaways
- The biologic hypothesis that the IDO/TDO pathway is immunosuppressive in cancer (through production of kynurenine) is strong and based on almost two decades of research
- Rushing into a phase 3 study from small uncontrolled early phase data comes with considerable risk of late stage failure
- Epacadostat shouldn’t necessarily be shelved right away; Incyte should take the time to confirm the right dose and who would best benefit (patient subsetting)
- TDO is expressed in addition to IDO in many tumors, including melanoma, and could make selective IDO inhibition insufficient to relieve the suppressive effect of kynurenine; IDO inhibition, at best, decreases kynurenine by 50% in serum
- Inhibiting downstream of IDO/TDO, where the pathways converge, would be a more potent way of impinging on this important pathway
Is epacadostat the right compound to test IDO as a target and was the right dose used for ECHO-301?
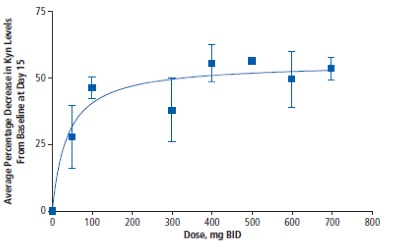
The short answer is – there remains some real doubt, but more data is needed. Michael Gladstone wrote about this question in a previous LifeSciVc post (here) but I will expand further. The pharmacodynamic data available is in the serum (not tumor) and shows a maximum reduction in kynurenine of 50% (see below). Given that kynurenine is synthesized by both IDO and TDO, a 50% reduction is what one would expect for complete IDO inhibition which is supported by preclinical studies. The phase 3 dose for ECHO-301 was 100 mg BID. As you can see from the graph, stable 50% inhibition isn’t seen until the 400 mg BID dose. Other PD data available was in an ex-vivo assay that is not sufficiently informative in my opinion. Therefore, the public PD data is spotty for epacadostat and it would be valuable to see a time course to know if the phase 3 dose is durable in order to judge if the right dose was chosen to maximize the possibility of seeing efficacy.
The BMS IDO inhibitor, BMS-986205, demonstrated a 50-60% reduction in serum kynurenine at trough on day 14 at the dose they are combining with nivolumab. The BMS PD data is more convincing than epacadostat in my opinion. It is also worth mentioning that epacadostat is an efflux substrate (PGP and BCRP) and therefore it would be important to see tumor PD given that melanoma is known to express these pumps. One of the key questions for IDO1 inhibitors is whether or not a 50% kynurenine reduction is sufficient for clinically relevant activity – more on that below.
How valid are single arm combination trials for decision making?
This particular clinical evolution feels like a salutary reminder of the advice “Be quick but don’t hurry.” The phase 1/2 epacadostat data showed an efficacy benefit over historical data with pembro alone (KN006). According to Incyte’s recent announcement the pembro alone arm in the ECHO-301 study replicated KN006. However, the PFS in the interim data was not improved and the hazard ratio (HR) was 1.000. What failed was the translation of efficacy from phase 2 to phase 3 – not the overperformance of the control arm (which has happened in other confirmatory phase 3 trials). Why this didn’t replicate is an open question and many companies have been running non-randomized IO trials for signal seeking, some of which lost significant value on the announcement of ECHO-301 data.
This raises the topic of how to most prudently progress agents with exciting early data – should all phase 2 trials for IO in combination be randomized going forward? This may be important for indications with higher rates of single-agent PD-1 responsiveness (like the IO-naïve melanoma patients in ECHO-301), but properly designed single-arm studies can still be very informative if the baseline activity of the combo partner is near zero in the target indicaiton. For example, anti-PD1/L1 agents have essentially no single agent activity in microsatellite-stable colorectal cancer (MSS CRC), so robust activity for a combo regimen is far easier to associate with the combo agent.
Does this negative result predict the outcome of other IDO trials?
The BMS IDO inhibitor and NewLink Genetic’s indoximod (an IDO pathway inhibitor with an unknown target) are being studied in combination with anti-PD1 in melanoma and it is now hard to imagine these trials showing efficacy unless these companies stratify the patients vs treating all-comers. For the other indications where there are ongoing phase 3 trials, which include non-small cell lung cancer, renal cell carcinoma, bladder, head and neck, and other cancers, I believe this comes down to the fundamental biology of IDO in those diseases that could be different than melanoma.
It is also worth remembering, Avastin’s first major attempt in breast cancer failed but Genentech pushed on and both Avastin and the anti-angiogenic class more broadly saw therapeutic success. I believe it is worth continuing with IDO trials in combination, but the focus must shift toward defining which patients stand to benefit based on immune cell and other changes in the tumor, as there are multiple variables here that need understanding.
Back to the basics and looking forward: what is next in the IDO/TDO pathway?
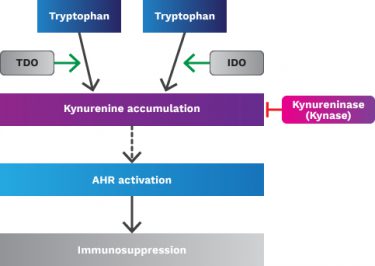
IDO was identified early on as an interferon response gene, which induced immunologic tolerance and acted as a negative regulator of T-cells. In most cases, high IDO expression is associated with poor clinical outcome, distant metastasis and decreased PFS. The effect of the pathway comes from accumulation of kynurenine (a product of tryptophan) that binds to the aryl hydrocarbon receptor (AHR) in immune cells causing broad immunosuppression. Indeed, a study by BMS and others at ASCO 2017 showed an increase in serum kynurenine in nivolumab-treated melanoma patients which correlated with lack of response to single agent nivolumab.
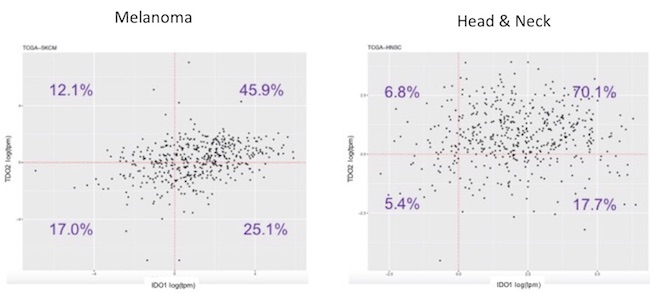
As mentioned above, data from epacadostat and the BMS IDO inhibitor demonstrates about a 50% kynurenine reduction in the serum of treated patients. BMS is the only company that published data on intratumoral PD with an IDO inhibitor, and their clinical data showed reduction in kynurenine in some but not all tumors. It is well known that the other kynurenine-producing enzyme, tryptophan dioxygenase (TDO), efficiently accumulates kynurenine from tryptophan. TDO is less studied than IDO but has recently garnered interest because of its overexpression in some tumors, including melanoma. We have done our own RNAseq analysis using The Cancer Genome Atlas and seen that TDO is overexpressed in tumor vs normal tissue and that it is expressed in tumors (including melanoma) by itself or with IDO (see below).
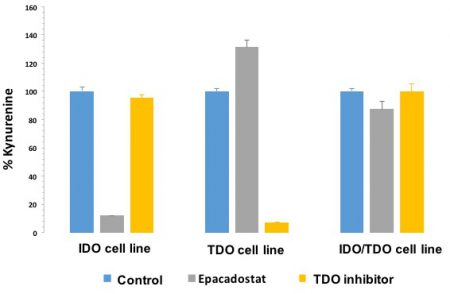
We know that when TDO is co-expressed with IDO that selective IDO inhibition (such as with epacadostat) does not decrease kynurenine levels (shown below). As many tumors co-express IDO and TDO (as the data above indicates in melanoma and head & neck cancer), IDO inhibition alone will probably not suffice in most cancers to prevent kynurenine-mediated immunosuppression.
IDO/TDO dual inhibitors are being explored, but none have advanced to the clinic so far. One concern with this approach is that dual inhibition would completely inhibit tryptophan metabolism through this pathway and potentially be toxic. This awaits experimental validation.
To circumvent this enzymatic redundancy, several companies are targeting kynurenine itself or this pathway’s ‘end game’ receptor, the aryl hydrocarbon receptor (AHR). Once again underscoring the diversity of immunosuppressive targets in play, AHR is a transcription factor whose up-regulated expression induces various tumor survival factors and reduces inflammatory cytokines, diverse outcomes which have been observed in multiple cancer types.
Kyn Therapeutics was established 2 years ago with the premise that attacking downsteam of IDO/TDO would be more effective than IDO selective inhibition. At Kyn we are developing 2 approached along this strategy. One is a therapeutic enzyme called kynureninase that degrades the metabolite and the other is a small-molecule antagonist against AHR. Two other companies are also developing AHR antagonists , Ideaya Biosciences and Hercules. The benefit of these approaches is to attack the pathway regardless if the tumor expresses IDO, TDO, or both. In addition, AHR may be activated by multiple ligands other than kynurenine.
While ECHO-301 was a definitive hit on IDO inhibition, too many fundamental biological aspects of this pathway have promise and need further investigation. Digging in to understand which target and which patients is a necessary step before rushing into advanced clinical studies with any of these approaches.
Thinking bigger than IDO
There are other immuno-metabolism pathways that have great potential, driving significant investment by/into several other companies who have been working in or just joined this space, including Agios, Kyn Therapeutics, Ideaya Biosciences, Rheos Therapeutics and Dracen Pharmaceuticals. There are many approaches now under investigation.
For example, the metabolism of other amino acids such as glutamine, arginine and particularly adenosine are receiving increased support, and glucose metabolism itself has been known to be harnessed by tumors for many years.
There are several companies targeting the adenosine pathway as this metabolite accumulates in the tumor microenvironment in inflammatory conditions generated by hypoxia. Adenosine is generated from ATP that is released from the cell by 2 cell surface enzymes (CD73 and CD39) that are also drug targets. Other drug targets include the ones on immune cells that promote the immunosuppression (A2A and A2B).
One relatively novel entrant to immuno-metabolism study is the COX-2/PGE2 pathway, which is known to interact with many immune cell subtypes including myeloid suppressor cells, regulator T cells, and macrophages. EP4 is a receptor for prostaglandin E2 (PGE2) and has been associated with immunosuppression. Increased EP4 activity is also linked to cell growth, angiogenesis, and metastasis. Strong preclinical data supports EP4 inhibition in combination with anti-PD1 and anti-CTLA4 agents.
The takeaway: follow evidence to conclusions, don’t jump to them
I’ve outlined several of the questions that people close to the tryptophan/kynurenine pathway are considering in the wake of the ECHO-301 study result. There are clear red flags on the path to the hype this study gathered, and hopefully we can collectively learn from that and emphasize both a) a more prudent approach to development of exciting early-stage assets, and b) a more cautious placement of expectations on the most advanced candidates with a novel target. Immuno-metabolism offers us a rich variety of pathways and targets and we are seeing a broad array of evidence coming to light across the field that promises many more potential targets for immunotherapeutic development in the years ahead.